
Small Molecule Manufacturing
- Process development & optimization
- cGMP API manufacturing
- High-potency compound handling (HPAPI)
- Controlled substance production
- Analytical development & stability testing
- Scale-up and tech transfer
Our chemical production lines are fully compliant with FDA, ICH, and global regulatory frameworks.
Medical Device Manufacturing
- Cleanroom manufacturing (ISO 7/ISO 8)
- Injection molding & precision component fabrication
- Device assembly & packaging
- Sterilization coordination (EtO, gamma, autoclave)
- Design verification & validation support (DV/V)
Our team helps clients reduce time-to-market while maintaining full adherence to ISO 13485.

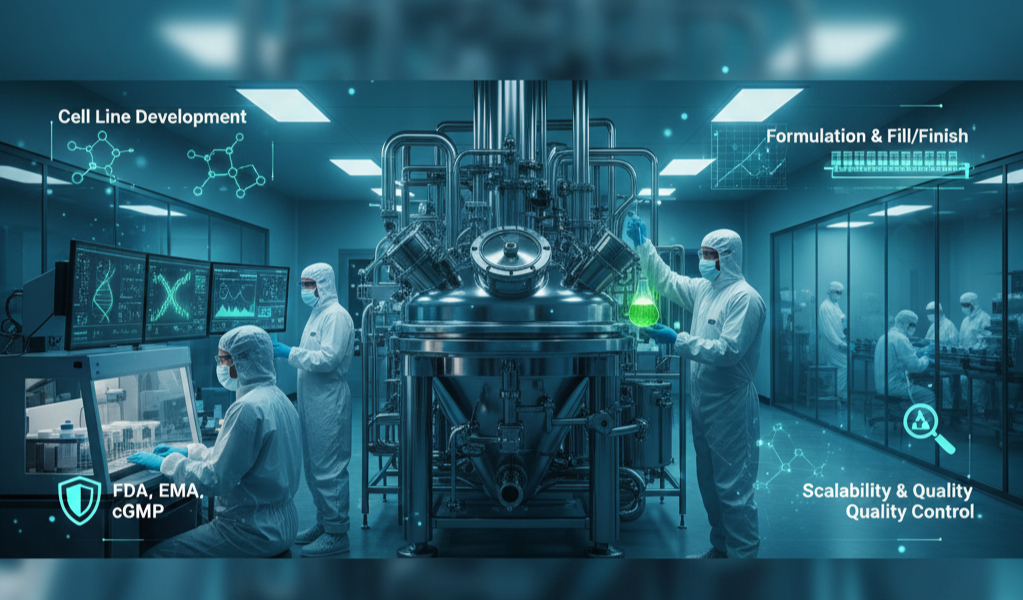
Biological Product Manufacturing
- Mammalian & microbial cell culture
- Monoclonal antibody (mAb) production
- Recombinant protein expression
- Viral vector development support
- Purification, formulation, & fill-finish
- QC release testing & GMP stability
We maintain robust contamination controls and scalable bioprocessing solutions.